by Drs. Mark J. Manhart, Thomas B. Steg, March, 2012
ABSTRACT
Clostridium difficle (C. difficle) is an uncommon strain of anaerobic, gram-positive spore-forming bacteria that gather in colonies and starve themselves for long periods of time while under antibiotic or chemo therapy. Then, when the medications have run their course and the environment once again favors the C. difficle spores, they germinate and reappear as C. difficle that grow to re-populate new colonies. These microbes are difficult to culture, grow slowly, release inflammatory toxins that damage mucosa of the colon, and spread by way of a rectal/oral pathway. Re-generated C. difficle can produce even more potent toxins responsible for some of the most serious human infections resistant to antibiotics. Their heat-resistant spores can persist for several months to years in their favored environment, all of which is reminiscent of the properties of Fusobacteria. The aim of this paper is to evaluate recent findings in light of forty years of human clinical experience in dentistry managing and controlling anaerobic bacteria with calcium materials.
BACKGROUND
C. difficle was identified in 1935 as a component of the fecal flora of healthy newborns and not thought to be a pathogen. Investigators noted that the bacterium produced a potent toxin. Its relationship to antibiotic-associated inflammations was more fully understood by the mid-1970s. Unfortunately, extended antibiotic use suppressed normal bacterial flora activity in the gut and actually supported the toxins released by C. difficle contributing to colitis in humans. The traditional antibiotic regimens for colitis have been shown to increase the risk of the disease, and cessation of the antibiotic becomes essential for the normal bacterial flora to recover within a few days. The problem has arisen that the antibiotics destroy so much of the varied bacterial content of the gut that the C. difficle soon governs the environment. Without the antibiotics to prevent a relapse of the colitis, an elongated therapy to “starve” the C. difficle spores may well be considered. Such a therapy would need to preserve the delicate balance of the normal bacterial activities of the digestive system.
Fully 25 years ago, Hawazin Foruki, et al [1] found that safe concentrations of ionic calcium in the external environment of C. difficille caused damage to its cellular membrane. This rendered the antibiotic-resistant pathogen more susceptible to breakdown. Around the same time, in 1987 Caspar, Florin and Thelestam [2] confirmed that the uptake of external calcium is needed to prevent toxic internalization through the healthy cell membranes. Then, Anne-Judith Waligro et al [3] in France conducted a remarkable study of how C. difficle spore attachment to healthy cell membranes can be modified. Calcium compounds were used to raise the pH of the environment. The French team showed that a calcium-rich medium under anaerobic conditions affected the C. difficle spore adhesion to culture cells to a considerable degree. The C.difficle spore adherence patterns are illustrated as shown in A and B Panels.
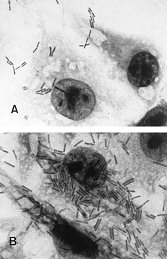
Patterns of C. difficile adherence to control cells examined by light microscopy (magnification x 1,000) in Panel A, C. difficle adherence treated at pH 7 (calcium); in Panel B, C. difficle adherence treated at pH 4 (control).
The C/ difficle spore adherence to cells within a calcium-rich medium (pH 7) was 8.3-fold less than that of a pH 4.0 standard control medium. Furthermore, Waligro noted, “The increase in adherence caused by an acidic pH could result from unmasking of a bacterial cell surface component…” That is, as in the Hawazin study, the cell membrane may be changed by its environment, and cell membrane adhesion by C. difficle spores is significantly reduced by a minimal inhibitory concentration of environmental calcium.
METHODS
We are trying to evaluate the oral cavity more fully as the very-well-connected entrance to “enteric biotherapy” at the commencement of digestive system. Therapy seems to have come full circle from dental implants to “fecal transplants” against colitis. The preferred path of colitis therapy is oral administration of diluted, filtered fecal matter. Fecal transplantation (fecal enema) has been reported to repopulate the colonic flora, but is limited by the risk of abdominal pain, hepatitis, weakness, fever, diarrhea, kidney failure, retrovirus transmission, colon surgery or death. Dr. Mark Rupp [4] points out that this therapy for intestinal infections has been around at least over 25 years, and those used against resistant C. difficle are 90% effective. The issue of administrating other bacterial preparations is under investigation. Some scientists are attempting to encapsulate the fecal dilution for patients to take orally.
Similar to the anaerobic Fusobacteria colonies that are specifically attracted to the colon and the periodontium, C. difficle is attracted by long-term, low-grade inflammation and has developed significant resistance to antibiotic therapy. Even when “good” bacteria are essential to digestion and retaining the “good” components of food, problems arise. And when confronted with “bad” bacteria, especially when they seem to work at times as partners within the same biotherome, complications can become exponential. Therefore, respect is essential toward any complex digestive medium that stimulates the production of vitamins, regulates the immune system, and enables anaerobic bacteria to “starve” themselves until the typical medical regimens used to destroy their diverse colonies are withdrawn. We have now studies of the oral-gut connections made by the apparently innocent vagus nerve. It provides timely communication with the intestinal tract in its brain/colon route visiting almost every major organ in between. The vagus also winds its way back to the brain bringing data from the tongue and throat. The slightest communication between these sensory feelings can express clearly our “gut feelings,” and this makes for serious concern for patients with colitis, several lower intestine inflammations, and dental pathologies.
Such evidence applied to similar anaerobes seems to reveal a dental connection with an eye to manage and control C. difficle as we have Fusobacteria. It is not our aim to kill either bacteria with powerful drugs, but to affect the environment making it difficult for them to replicate and leave them susceptible to cellular breakdown without harmful collateral damage. This may be accomplished with applications of calcium materials. Over four decades we developed nonsurgical root canal therapy with calcium materials. Shortly thereafter, we designed a nonsurgical periodontal therapy utilizing timed-release calcium compounds to affect the oral environment for extended periods. The therapy not only controls the dreaded periodontal disease, but also exposes the true causes of the infection, and with a little patience, eliminates the causes without antibiotics and without destroying the very cells needed for recovery. These direct, general, and spot-applications against oral infections are rapid in their response.
We early on realized that periodontal disease is the result of something, not the cause, and the causes are not always easy to eliminate. When a persistent approach toward controlling the environment is taken, the healthy cells “explode” in their normal fashion of regeneration. This has shown to be very much the clinical experience with calcium therapy. They are very effective when used against anaerobic infections of dentition or bone tissue in consistent monthly treatments over 10 to 12 months. In cases of soft tissue infections successful regimens are shorter, but still required 10 to 12 weekly treatments over three months. Factoring in the number of applications within a projected reduction rate of ten percent, this has been an acceptable expectation for healing. Then, the special issue of “diastemic dilemma” appeared. [5]
It is routinely claimed that the diastema condition is merely a spacing of the upper central incisors traditionally attributed to “genetic deviation.” That would be a non-sequitur, that is:
“An argument in which its conclusion does not follow from its premises, and the conclusion could be either true or false, but the argument is fallacious because there is a disconnection between the premise and the conclusion.”
On the one hand, we are still taught to this day that the central incisors just erupted in the wrong place. They do quite often, and it is commonly proscribed that all we have to do is move them back with orthodontic braces, or restore their cosmetic elegance and position with fillings or crowns. The spacing of those incisors is not a genetic accident. It is unilateral deviation caused by a pathological cyst harboring Fusobacteria and their toxins in the bone on the maxillary midline between the roots of the central teeth. This infection most often kills one of those centrals. One the other hand, authentic clinical experience over fifty years reveals a far more valuable reality.
Consequently, when faced with the wisdom of common sense, we realized this makes perfect sense for several reasons. The foremost would be, with very little effort or time, we find a dead tooth and a cyst the size of an almond looking right at us. As soon as possible non-surgical endodontic root canal therapy is undertaken on the non-vital central, and calcium materials are applied to the periodontium of maxillary anterior quadrant . Over the course 10 to 12 calcium treatments, the symptoms that were inadvertently hidden and tolerated for years or decades, quickly dissipate. Reflecting on the manner in which calcium affects pH and oxygen levels of the digestive environments, it is understandable how difficult it is to control these anaerobic strains with antibiotics. However, to us it has made positive and assertive common sense to use timed-release calcium materials in the extracellular environment to protect cell membranes against the adhesion of these Fusobacteria and C. difficle spores at the outset of the enteric system as well as at its conclusion.
DISCUSSION
Difficulties are a great deal more challenging knowing the digestive tract as a puzzling enigma with all its variety of bacterial strains, potential hydrogen concentrations, and oxygen levels. The lower the pH, the more acidic, and the less oxygen there is in the environment. The higher the pH, the more alkaline, the more oxygen there is in the system, the pH of 7.0 being neutral. Healthy human blood (pH 7.4) and bacteria must maintain narrow pH ranges around 7.35 to 7.45 pH. Caught in an alkaline range, anaerobes of the Fusobacteria and C. difficle variety must starve themselves and reduce their rate of replication against an oxygen-enriched condition or to outlast antibiotic therapy. And yet, those factors also appear evident in the success of calcium use in periodontal and root canal therapy. In fact, they seem to explain the critical need for higher pH concentrations of almost a year’s duration to elicit success in Osteo-Endo-Cystic Therapy [5]. An example is the need anaerobic bacteria have to remain free of oxygen exposure. They are obligatory anaerobes and die in sustained oxygenated environments. And, their spores are significantly less capable of adherence to cell membranes in the presence of calcium.
It has been well known that calcium materials supply abundant amounts of free calcium to the healing process of dental tissues. It raises the environmental pH well into the alkaline range. Some calcium compounds will safely sustain significant readings above the baseline of Ph 7.4 for three days to nearly a week without untoward affects to healthy tissues. Therefore, it may not be surprising that if each treatment reduces the rate of replication of the offending bacteria ten percent, complete destruction of the infection, may occur after 10 to12 treatments. This has been demonstrated in hundreds of severe clinical cases. Nonetheless, it is yet unclear whether the most successful dosage should be weekly or monthly.
More recent studies in dealing with deviations of these central incisors, the foci of Fusobacteria colonies, and the “self-starving” properties of anaerobic C. difficle and Fusobacterium may open doors for advancement. The calcium approach is to lay “siege” to bacterial ingenuity. That is, find them, control their environment with calcium, manage their replication with oxygen, protect the healthy tissues, and outlive the rascals. Destroying “bad” organisms in milliseconds may be popular or necessary, but if calcium does reduce anaerobic bacteria replication so readily over enough time, the healthy tissues will recover with fresh oxygenation, dominate the field, and regenerate naturally. This approach quickly becomes more than mere survival of the fittest, but survival with less anaerobic toxicity.
CONCLUSION
This paper reflects contributions to that realistic possibility. Don’t kill them with drug therapy, but take advantage of oral administration and resolve both problems through the mouth. We have had experience with thousands of calcium treatments against “bad” oral pathogens to fuel cysts that destroy teeth, cause diastemic spacing, and as we have seen over years, result in complete dental breakdown. There are only two areas in the body where Fusobacteria colonize, the colon and the oral cavity. C.difficle favors the gut with its anaerobic colonies and is powerfully affected by oral doses of enteric biotherapy. There has been real and palatable affects from calcium therapy on infections the oral cavity, and quite possibly, on the lower intestinal tissues without problematic drugs, radical surgeries, or fecal transplants. More research linking these calcium methods to control C. difficle warrants serious consideration.
BIBLIOGRAPHY
1. Hawazin Foruki, et al; Effects of calcium on in-vitro activity against Clostridium difficle, American Society for Biology, March 1987
2. Maria Caspar, Inger Florin, Monica Thelestam; Calcium and calmodulin in cellular intoxication with Clostridium difficle toxin B, Journal of Cellular Physiology, July 1987, online February 2005
3. Anne-Judith Waligro, Maria-Claude Barc, Pierre Bourlioux, Anne Collignon, Tuomo Karjalainem; Clostridium difficle cell attachment is modified by environmental factors,
Universite de Paris-Sud, France, Allied and Environmental Microbiology, September 1999
4. Dr. Mark Rupp, Chief ofj Infectious Diseases, University of Nebraska Medical Center, March 2013
5. Mark J. Manhart, Thomas B. Steg, Calcium Method of Osteo-Endo-Cystic Therapy, accepted by the Oral Clinical Investigations in December 2008, published and presented at ConsEuro Dental Congress, Seville, Spain, March 2009, on line Calcium Therapy Institute, January 2011
