by Mark J. Manhart DDS, Thomas B. Steg DDS
October 2011
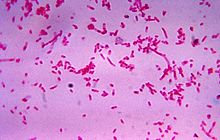
Fusobacteria are also parasitic, motile and contribute to several infections beyond periodontal disease including the rare “sore throat” Lemierre Syndrome that results in pus pockets on the tonsils, and topical skin ulcers, reported G. Weinstock.[2] They not only flourish in these tissues, but easily flit about on other cells to similar vacation spots around the body accounting for the spread of their acidic toxins, at times arousing havoc with tumors, polyps, cysts and other harmless masses of tissue. Even a change of environment with local inflammation can establish a predisposition for excessive growth of functionless tissue. S.H. Aliyu, R.K. Marriott, and M.D. Curran [3] found that the oropharanx is rarely invaded by Fusobacterium. But according to A. Park, [4] the lower intestine and rectal areas are susceptible to this parasitic microbe.
A characteristic of slow-growing infection or dense tumors around the mouth and rectal areas is that microbes outnumber healthy human cells more than expected. [5] RA Holt’s initial finding in colon infection was that Fusobacteria outnumbered human cells by a ratio of nine to one. However, with a more accurate genetic test probing for cells near ulcerous colon cells, his RNA analysis revealed colon cancer had an average of 79 times as many Fusobacteria as normal cells. In even more sensitive tests probing for Fusobacteria genes, Holt found an average of 415 times as many indicators of Fusobacteria in the tumor cells as in normal cells. There is an abundance of Fusobacteria in tumors, but they are not among the prominent species in the digestive system. Nonetheless, they do elicit inflammation which attracts specific organisms, some of which aggressively invade the damaged tissues. Fusobacteria congregate around chronically inflamed cells and burrow into the tumor or cyst while discharging their acidic toxins that affect the surrounding environment.
Beyond that, D. Relman notes that some organisms cause inflammation themselves and become a good place for an anaerobic pathogen to set up shop and replicate at its leisure. [6] Often what distinguishes disease-causing cells from harmless cells is an ability to invade other cells, and especially when given a friendly environment. His studies lend credence to the notion that it doesn’t matter what created the inflammation, the cells can spread invasive Fusobacteria to remote tissues.
METHODS and MATERIALS
From a 45-year history of clinical studies and practices, the Calcium Therapy Institute has
designed non-surgical calcium modalities and formulated calcium materials that manage, control
and stimulate healing in endodontic root canal sealing, root trifurcation infections, subgingival
plaque and calculus inflammations, prolonged oral mucosa and skin inflammations, and in longlasting elevation of calcium levels in blood serum. These therapies range from local to fullblown complex ailments of both the mouth and the skin. [7] The properties of the materials are anti-inflammatory, antimicrobial, alkaline, timed-release for prolonged action, and both soft and hard tissue-friendly.
More recently, M.J. Manhart and T.B. Steg report non-surgical calcium treatments for bony cysts in the maxilla related to diastemic deviations and necrosis of a central incisor, that is, osteoendo-cystic therapy. Their findings date back 35 years in hundreds of cases. The three infectious phases slowly combine from rudimentary cells forming the incisive canal of the maxilla, the inflammatory pulpal necrosis of the adjacent central, and the periapical abscess in the bone. This long-term condition would assuredly attract Fusobacteria from the oral cavity, especially when initiated by any one of a number of incidents like periodontal flare-ups, traumatic dental injury, extensive dental restoration procedures or orthodontic appliances. Several other are still suspect. The resultant toxins seek drainage by an easy route, into the mouth down the incisive canal and emptying onto the palate lingual to the centrals and directly onto the mandibular incisors. With little effort this situation results not only in a constant acidic flow reducing the buffer capacity of the saliva for the entire mouth and the immediate gastro-intestinal tract. Most often, years of this compromised environment renders a maxillary central incisor necrotic by the shear presence of aggressive burrowing pathogens such as Fusobacteria.
If the anaerobe Fusobacterium resides most often in the oral cavity, in dental plaque, and is linked to periodontal disease and inflammatory gingivitis, then the next most favorable home for Fusobacteria evidently is the lower intestine to spur on ulcerative colitis, cancer and Crohn’s disease. Both regions, the mouth and rectum, can be easily inflicted with abnormal levels of Fusobacteria which create inflammatory environments for long periods of time and enhance the spread or the distribution of cells, even to distant organs of the body that are notorious for harboring anaerobic microbes. It is well known that biofilm colonies readilycause cystic and abscess infections.
A reason for this scattering of anaerobes would be that Fusobacteria gain a stable foothold in the maxilla to proliferate and discharge their flood of toxins into the porous maxillary bone, onto the palate, and lower dentition. Furthermore, this process is plugged into the normal eating, breathing, speaking and sucking mechanisms of the body, It is a perfect parasitic relationship for the unusual imbalance of Fusobacteria to human cells. In dental infections overload of pathogens becomes voluminous and touches all ages. The most striking case is the space between the maxillary centrals, that is, diastemic spacing or deviation. The cystic infection over decades gradually moves incisors, almost exclusively unilaterally, out of place or in some deviated manner, all the while leaching the life out of the teeth involved and invading the lower anterior segment of teeth and their periodontal structures, even after the teeth have been removed.
MANAGEMENT
A recent breakthrough is reported by D. Ngyen [8] and her North American researchers. Their years of research have broad implications for many anaerobic bacteria that, attracted by local irritation or inflammation, turn on a “starvation trigger” that signals the aggressive bacteria to release an enzyme to reduce the affects of antibiotics. This “starvation signal” by self-starving bacteria produces a state of hibernation, or defense as it were, until the antimicrobial medications are withdrawn. They become dormant, only to return on the offensive in a more favorable climate.
Nygen’s question is: How do we turn off starvation alarm signals? Discovery of the biological properties of salivary peptides from the submandibular gland under the tongue, along with the calcium materials used in root canals and periodontal therapies contribute depth to the answer. They are non-steroids with antipyretic, anti-inflammatory, timed-release properties. Several clinical studies have suggested that a prolonged intake of salivary gland secretions and tissuefriendly calcium materials have positive systemic effects.
In the mid-1980s Manhart’s blood serum study [9] revealed that certain calcium materials, , did not kill bacteria but “turned down” their replication rate, while popular antimicrobial agents killed everything in milliseconds, the healthy cells and bacteria, so fast that the Time could not be recorded. In that study, and from years of clinical observations of calcium treatments to oral tissues, it took ten hours to eliminate the bacteria. The critical factor was Time. In light of the starving bacteria findings, calcium materials leave the starvation alarm On, and yet, do no harm. For decades the calcium therapy has shown to be harmless to any normal healthy cells of the oral cavity or the skin, while they sustain an environmental “siege.” Not kill them outright, but gradually starve the starving bacteria to death.
Yes, not kill, but starve them. So, the question to ask is, How do you turn Off the alarm of bacteria for a long time, without harm to other tissues? The calcium answer is, “Don’t. Keep the alarm On.” Be parsimonious, be mercenary. Starve the “Staving Old Bacteria.” Outliving them takes Time, but this need not cause us undue anxiety. Bacteria starve themselves rapidly, so keep them famished, destitute, and on the commons until they no longer have an appetite to alert the biofilm colony. Then, with little ability to replicate, they proceed to perish, while the healthy cells quickly flourish and dominate the field, especially with a timed-release calcium protagonist.
This ever-present process of feast or famine is illustrated clearly with the 10-to-12 month recovery process that apical and periapical tissues of teeth must undergo after endodontic therapy. All may seem well and good, but Time is still the critical issue. Thus, Reason need not be stretched to expect cystic infections that cause deviations and diastemic spacing of the maxillary anterior dentition, which by the way, took 20 to 30 years to damage the bone and kill one of the incisors, to take 10-to-12 calcium treatments over a year to heal. Problems arise when therapy is spasmodic or terminated too soon. Again persistence is essential, especially in the face of what appears to be complete recovery. That is, without harm, turn down their growth, starve the starving bacteria to death, and watch the recovery. It seems to be a secure implication that many bacterial infections respond in this manor to calcium materials.
Lengthy case reports by the Calcium Therapy Institute indicate an ever-present level of infection directly connected to the diastema deviations. In virtually every case these displacements are due to a midline cyst between the roots of the maxillary centrals, one of which suffers complete necrosis in the process of being shoved out of place by the bacterial invasion of a relentless, encapsulated cyst in the maxilla. The cyst kills the tooth, or some tooth damage initiated the cyst. In some cases the cause may be unclear, but the symptoms soon tell the story. Collateral damage to the anterior dentition is far too common to be accidental. These may be common abnormalities, but not innocent. In fact, when these protracted disease-causing conditions are allowed to progress into middle age, they likely account for and push on complete dental breakdown. Meanwhile, calcium materials have shown to be remarkable treatments that manage inflammation, reduce infectious environments, and stimulate the regeneration of damaged soft tissues and bone.
The management of Fusobacteria and neutralizing their toxins remains within the scope of controlling the environment of anaerobic microbes like Fusobacteria. Similarly, a major consideration of health is to restrict local and systemic spread of invasive pathogens. It is evident from managing the environment of the mouth with calcium materials results in rapid healing and reduction of plaque deposition on the mandibular anterior incisors. Patients who have undergone extended calcium treatments of oral mucosa and the periodontal tissues experience a significant reduction of plaque and calculus.
Considering the two most predominate gathering places for Fusobacterium, the oral cavity and the colon, it would seem appropriate to continued research on calcium materials. It appears this pathogen can be managed far more readily than expected, even in the early stages of inflammation and infection of the mouth and the lower digestive tract.
BIBLIOGRAPHY
1. M. Meyerson, Dana-Farber Cancer Institute, Boston, MA
2. G. Weinstock, University of Adelaide, Australia
3. S.H Aliyu, R.K. Marriott, M.D. Curran, J. Med. Microbiol. 53
4. A. Park, Time.com 2011
5. R.A. Holt, British Columbia Cancer Agency
6. D. Relman, Stanford University, CA
7. M.J. Manhart, T.B. Steg. Calcium Therapy Institute, Omaha, NE
8. Dao Nyugen, et al, Science Magazine 12-2011, Canada
9. M.J. Manhart, T.B. Steg, Fusobacteria Management, Omaha, NE
